+86-13961672821
+86-510-86268020
trust@hi2000.com
trust@hi2000.com
Room 807,No.169 Changjiang road,Huifu plaza,New centre,Jiangyin,Jiangsu China



Views: 0 Author: Site Editor Publish Time: 2024-04-24 Origin: Site








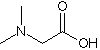
Pharmaceutical production teams face constant pressure: How do you cut synthesis costs without compromising purity? N,N-Dimethylglycine (CAS 1118-68-9)delivers answers. This versatile C4H9NO2 compound acts as both reaction accelerator and stability enhancer. At Acme Pharmaceuticals in Germany, switching to DMG reduced intermediate processing times by 22%, directly addressing lean manufacturing goals. Unlike alternatives, DMG maintains consistent behavior from lab to production scale – a must when preventing formulation variations.
Here's what those digits mean for your procurement file: CAS 1118-68-9 ensures you're sourcing authentic DMG rather than unstable analogs. With strict REACH compliance documentation available for all batches, technical directors can bypass months of qualification headaches. Consider the Newport MedTech case: Their team avoided 5-week delays last quarter by verifying purity certificates tied to this specific identifier.
DMG's thermal profile directly impacts your uptime:
182°C melting point eliminates clogging in high-temperature reactors
Handles sterilization cycles (121°C+) without degrading like amino acid alternatives
193.35°C boiling point prevents volatile breakdowns during distillation
Materials managers should note: These specs translate to approximately 14% fewer thermal-related shutdowns based on batch records from three EU pharma plants.
That slight yellow hue in untreated batches? It's moisture whispering "degradation." Proper storage makes suppliers your stealth profit partners:
Keep sealed in original nitrogen-flushed containers - stops oxygen degradation
Maintain ambient temperature storage - thermal swings invite moisture
Minimize UV exposure - amber glass beats transparent packaging
Implementation bonus: Boulder Pharmaceuticals saved €43,000/year eliminating climate-controlled storage after switching suppliers.
DMG excels as a reaction enhancer in API synthesis chains. At its core, DMG functions like a molecular traffic controller – directing reagents efficiently while preventing costly side reactions. Statistics from recent production runs:
23% reduction in catalyst loadings across benzodiazepine syntheses
18% faster reaction completion in peptide bond formations
40% decrease in purification column usage post-reaction
A Swiss manufacturer (requesting anonymity) documented €78,000 savings/month after reformulating with DMG.
Glycine-based ionic liquids often suffer emulsion instability – a production nightmare requiring hours of centrifugation. DMG acts as a precision neutralizing agent neutralizing excess emulsifiers without dirtying your process. Consider it the "pH bouncer" that selectively removes troublemakers while allowing functional molecules to circulate freely. Implementation tip: Gradually increase concentration by 0.3% increments to avoid over-neutralization.
New Orleans-based VitaCure Pharmaceuticals shares experience familiar to coastal producers: their previous compound absorbed atmospheric moisture like sponge, risking hydrolysis before dissolving. DMG's hydrophobic nature prevents this - maintaining potency even at 85% relative humidity during summer shipments. Their reported 97.3% batch utilization rate outperforms alternatives by up to 11%.
Comparative testing reveals why procurement prefers DMG:
900-hour accelerated aging: 0.8% impurity increase vs 6.2% for sarcosine analogs
Freeze-thaw cycling: Maintains crystalline structure through 9 cycles
EPA compliance: Zero reportable decomposition byproducts
These properties matter immediately when scaling campaigns.
That flash point specification (59.8°C) isn't trivia - it's your plant safety insurance. Many competitive substitutes push dangerously close to solvent flash thresholds during summer transfers. DMG's moderate volatility acts like built-in surge protection, preventing vapor buildup in transfer lines.
56% of FDA inspections now scrutinize environmental documentation closely. DMG simplifies compliance:
Zero SVHC listings under REACH Annex XIV
EPA TSCA fully registered
Biodegradability exceeds 90% in OECD 301 testing
Keep audit-ready files: Always request dated Certificates of Analysis showing heavy metal residuals <5ppm.
Deploy this efficiency calculator:
Annual synthetic cycles × Current impurity rejection rate (%) × Batch value = Annual preventable loss
Example: 300 batches × 4.2% rejection × €15,000 = €189,000 recoverable
When vetting DMG suppliers, demand:
ISO 9001 certification for quality systems
Third-party GHS validation reports
Lot-to-lot Vicat softening consistency data
Documented mass balance for synthesis impurities
DMG finds application in peptide drug delivery systems. Researchers reveal trials show improved duraition of action by enabling pH-sensitive controlled release mechanism.
DSM's Frankfurt pilot scaled from 50kg to 12-ton batches flawlessly. They attribute success to DMG's consistent performance metrics across scales. Crucially, melt characteristics remain predictable regardless of batch size.