+86-13961672821
+86-510-86268020
trust@hi2000.com
trust@hi2000.com
Room 807,No.169 Changjiang road,Huifu plaza,New centre,Jiangyin,Jiangsu China



| Availability: | |
|---|---|








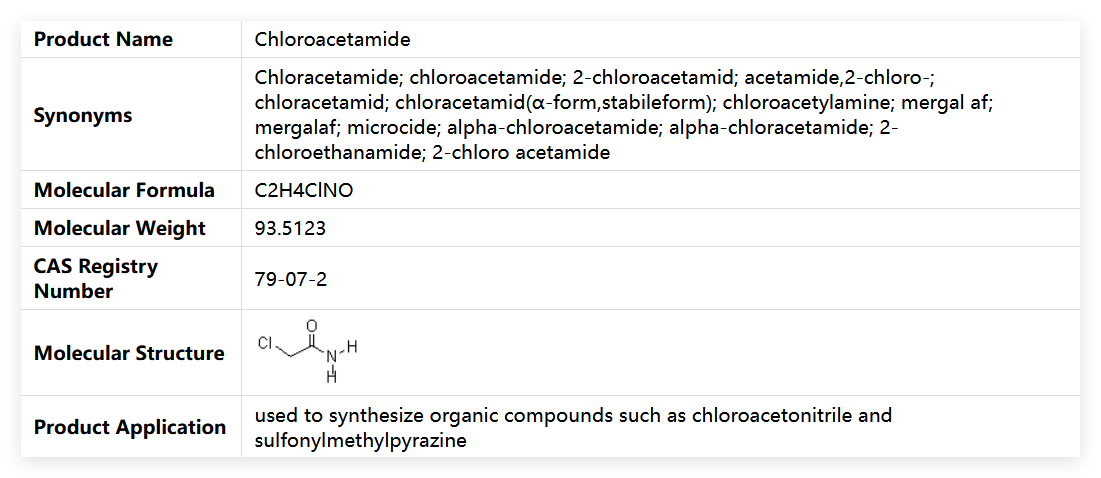
Chloroacetamide is an organic compound with a clear chemical structure and stable physicochemical properties, presenting as white crystals. With its unique molecular composition (molecular formula: C₂H₄ClNO; molecular weight: 93.512), it possesses multiple functional attributes such as herbicidal, bactericidal, and anti-corrosion effects. As a commonly used functional chemical in industrial and agricultural fields, it has a unique CAS number (79-07-2) as its identification, and also goes by aliases like "2-Chloroacetamide" and "chloroacetic acid amide". High-purity finished products can be obtained through standardized processes. It can exert effects stably under the premise of strictly following storage and usage specifications, but its toxicity and sensitization must be focused on to ensure safe operation.
The density is 1.3±0.1 g/cm³, and the melting point stably ranges from 116 to 118°C (literature-verified value); some experimental data show it can reach 119 to 120°C, with 116 to 118°C used as the main reference in practical applications. The boiling point is up to 256.0±13.0°C (under 760 mmHg), and the flash point is 108.6±19.8°C. It has low volatility (vapor pressure is only 0.07 hPa at 20°C), facilitating normal-temperature storage and transportation. In terms of solubility, its water solubility is 90 g/L at 20°C; it is soluble in ethanol and very slightly soluble in ether, which can meet dissolution needs in different scenarios.
Its core mechanism of action is to inhibit the "very-long-chain fatty acid elongase" in organisms and interfere with the fatty acid metabolism process. This characteristic makes it highly effective in herbicidal and bactericidal fields; meanwhile, as a preservative, it can effectively inhibit microbial growth and extend the shelf life of products.
It has acute toxicity (oral LD50 as low as 31 mg/kg, belonging to the highly toxic level), skin sensitization, reproductive toxicity, and aquatic biotoxicity. Its GHS symbols are GHS06 (Acute Toxicity) and GHS08 (Reproductive Toxicity), with the signal word "Danger". Strict compliance with protection specifications is required during use and storage, and contact with strong oxidants, strong acids, strong alkalis, and strong reducing agents should be avoided.
Suitable for various farmland scenarios such as dry land and paddy fields. As a herbicide, it can specifically inhibit weed growth and reduce nutrient competition between weeds and crops; meanwhile, as a fungicide, it can prevent common diseases of crops and reduce the risk of yield reduction caused by diseases. It is widely applicable to the planting needs of grain crops and cash crops, and its related effects have been verified by authoritative journal literatures such as *Agricultural and Biological Chemistry*.
When added in the production of glue, paint, and coating, it can effectively inhibit the growth of microorganisms (such as bacteria and mold), prevent product deterioration, mildew, and performance degradation during storage and use, and extend the shelf life and service life of products. It is applicable to various industrial product types such as architectural coatings, industrial glue, and furniture paint.
Due to its clear biological activity (interfering with fatty acid metabolism and acting on bacteria-related signaling pathways), it is used as a reagent in scientific research in the direction of "infection prevention and control". It serves as an auxiliary reagent for studying the function of very-long-chain fatty acid elongase and bacterial metabolism mechanism, and has been cited in 37 literature reviews, providing support for research in related fields.
- UN Dangerous Goods Number: 2811
- Transport Hazard Class: Class 6.1 (Toxic Substances)
- Packing Group: Group III (packaging for mild hazards, conforming to international transportation standards)
- Proper Shipping Name: Toxic Solid, Organic, N.O.S. (2-Chloroacetamide)
Double-layer packaging of "inner-lined plastic bag + outer wooden case/cardboard drum" is adopted. The inner-lined bag ensures tightness to prevent dust leakage; the outer case/drum must have a certain compression resistance to avoid damage during transportation. GHS hazard symbols, UN number, product name, and production information should be clearly marked on the packaging surface.
- Mixed transportation with strong oxidants, strong acids, strong alkalis, and strong reducing agents is prohibited to avoid chemical reactions leading to hazards;
- During transportation, sun protection and moisture prevention are required, and the carriage should be kept cool and ventilated, away from heat sources and fire sources;
- Handle with care during loading and unloading to avoid packaging damage; in case of leakage, immediately isolate the leakage area, and operators should wear protective equipment such as respirators and nitrile rubber gloves to collect the leaked material into a sealed container, and it is prohibited to discharge it into sewers or the environment;
- International transportation must comply with relevant regulations for maritime and air transportation (such as IMDG Code, IATA DGR). It is not a "maritime pollutant", but the toxic substance attribute must be declared in advance.
Q1: Is Chloroacetamide toxic?
A1: Chloroacetamide is toxic and can cause harm if ingested or inhaled. Proper safety measures should be followed during handling.
Q2: How should Chloroacetamide be disposed of?
A2: Chloroacetamide should be disposed of according to local regulations for toxic substances. It must not be poured down drains or mixed with other chemicals unless specified.
Q3: What safety precautions should be followed when handling Chloroacetamide?
A3: Chloroacetamide is an irritant and corrosive. Wear protective gear like gloves, goggles, and masks. Always handle it in a well-ventilated area.
Q4: Can Chloroacetamide be used in pharmaceuticals?
A4: Yes, Chloroacetamide is used in the synthesis of pharmaceutical intermediates and plays a role in producing various bioactive compounds.
Q5: What are the potential hazards of Chloroacetamide?
A5: Chloroacetamide can cause skin irritation and eye damage. It may also irritate the respiratory system if inhaled.
Q6: Can Chloroacetamide be combined with other chemicals?
A6: Chloroacetamide should not be mixed with strong oxidizers or alkalines. Care must be taken to avoid unwanted chemical reactions.

Chloroacetamide is an organic compound with a clear chemical structure and stable physicochemical properties, presenting as white crystals. With its unique molecular composition (molecular formula: C₂H₄ClNO; molecular weight: 93.512), it possesses multiple functional attributes such as herbicidal, bactericidal, and anti-corrosion effects. As a commonly used functional chemical in industrial and agricultural fields, it has a unique CAS number (79-07-2) as its identification, and also goes by aliases like "2-Chloroacetamide" and "chloroacetic acid amide". High-purity finished products can be obtained through standardized processes. It can exert effects stably under the premise of strictly following storage and usage specifications, but its toxicity and sensitization must be focused on to ensure safe operation.
The density is 1.3±0.1 g/cm³, and the melting point stably ranges from 116 to 118°C (literature-verified value); some experimental data show it can reach 119 to 120°C, with 116 to 118°C used as the main reference in practical applications. The boiling point is up to 256.0±13.0°C (under 760 mmHg), and the flash point is 108.6±19.8°C. It has low volatility (vapor pressure is only 0.07 hPa at 20°C), facilitating normal-temperature storage and transportation. In terms of solubility, its water solubility is 90 g/L at 20°C; it is soluble in ethanol and very slightly soluble in ether, which can meet dissolution needs in different scenarios.
Its core mechanism of action is to inhibit the "very-long-chain fatty acid elongase" in organisms and interfere with the fatty acid metabolism process. This characteristic makes it highly effective in herbicidal and bactericidal fields; meanwhile, as a preservative, it can effectively inhibit microbial growth and extend the shelf life of products.
It has acute toxicity (oral LD50 as low as 31 mg/kg, belonging to the highly toxic level), skin sensitization, reproductive toxicity, and aquatic biotoxicity. Its GHS symbols are GHS06 (Acute Toxicity) and GHS08 (Reproductive Toxicity), with the signal word "Danger". Strict compliance with protection specifications is required during use and storage, and contact with strong oxidants, strong acids, strong alkalis, and strong reducing agents should be avoided.
Suitable for various farmland scenarios such as dry land and paddy fields. As a herbicide, it can specifically inhibit weed growth and reduce nutrient competition between weeds and crops; meanwhile, as a fungicide, it can prevent common diseases of crops and reduce the risk of yield reduction caused by diseases. It is widely applicable to the planting needs of grain crops and cash crops, and its related effects have been verified by authoritative journal literatures such as *Agricultural and Biological Chemistry*.
When added in the production of glue, paint, and coating, it can effectively inhibit the growth of microorganisms (such as bacteria and mold), prevent product deterioration, mildew, and performance degradation during storage and use, and extend the shelf life and service life of products. It is applicable to various industrial product types such as architectural coatings, industrial glue, and furniture paint.
Due to its clear biological activity (interfering with fatty acid metabolism and acting on bacteria-related signaling pathways), it is used as a reagent in scientific research in the direction of "infection prevention and control". It serves as an auxiliary reagent for studying the function of very-long-chain fatty acid elongase and bacterial metabolism mechanism, and has been cited in 37 literature reviews, providing support for research in related fields.
- UN Dangerous Goods Number: 2811
- Transport Hazard Class: Class 6.1 (Toxic Substances)
- Packing Group: Group III (packaging for mild hazards, conforming to international transportation standards)
- Proper Shipping Name: Toxic Solid, Organic, N.O.S. (2-Chloroacetamide)
Double-layer packaging of "inner-lined plastic bag + outer wooden case/cardboard drum" is adopted. The inner-lined bag ensures tightness to prevent dust leakage; the outer case/drum must have a certain compression resistance to avoid damage during transportation. GHS hazard symbols, UN number, product name, and production information should be clearly marked on the packaging surface.
- Mixed transportation with strong oxidants, strong acids, strong alkalis, and strong reducing agents is prohibited to avoid chemical reactions leading to hazards;
- During transportation, sun protection and moisture prevention are required, and the carriage should be kept cool and ventilated, away from heat sources and fire sources;
- Handle with care during loading and unloading to avoid packaging damage; in case of leakage, immediately isolate the leakage area, and operators should wear protective equipment such as respirators and nitrile rubber gloves to collect the leaked material into a sealed container, and it is prohibited to discharge it into sewers or the environment;
- International transportation must comply with relevant regulations for maritime and air transportation (such as IMDG Code, IATA DGR). It is not a "maritime pollutant", but the toxic substance attribute must be declared in advance.
Q1: Is Chloroacetamide toxic?
A1: Chloroacetamide is toxic and can cause harm if ingested or inhaled. Proper safety measures should be followed during handling.
Q2: How should Chloroacetamide be disposed of?
A2: Chloroacetamide should be disposed of according to local regulations for toxic substances. It must not be poured down drains or mixed with other chemicals unless specified.
Q3: What safety precautions should be followed when handling Chloroacetamide?
A3: Chloroacetamide is an irritant and corrosive. Wear protective gear like gloves, goggles, and masks. Always handle it in a well-ventilated area.
Q4: Can Chloroacetamide be used in pharmaceuticals?
A4: Yes, Chloroacetamide is used in the synthesis of pharmaceutical intermediates and plays a role in producing various bioactive compounds.
Q5: What are the potential hazards of Chloroacetamide?
A5: Chloroacetamide can cause skin irritation and eye damage. It may also irritate the respiratory system if inhaled.
Q6: Can Chloroacetamide be combined with other chemicals?
A6: Chloroacetamide should not be mixed with strong oxidizers or alkalines. Care must be taken to avoid unwanted chemical reactions.