+86-13961672821
+86-510-86268020
trust@hi2000.com
trust@hi2000.com
Room 807,No.169 Changjiang road,Huifu plaza,New centre,Jiangyin,Jiangsu China



| Availability: | |
|---|---|








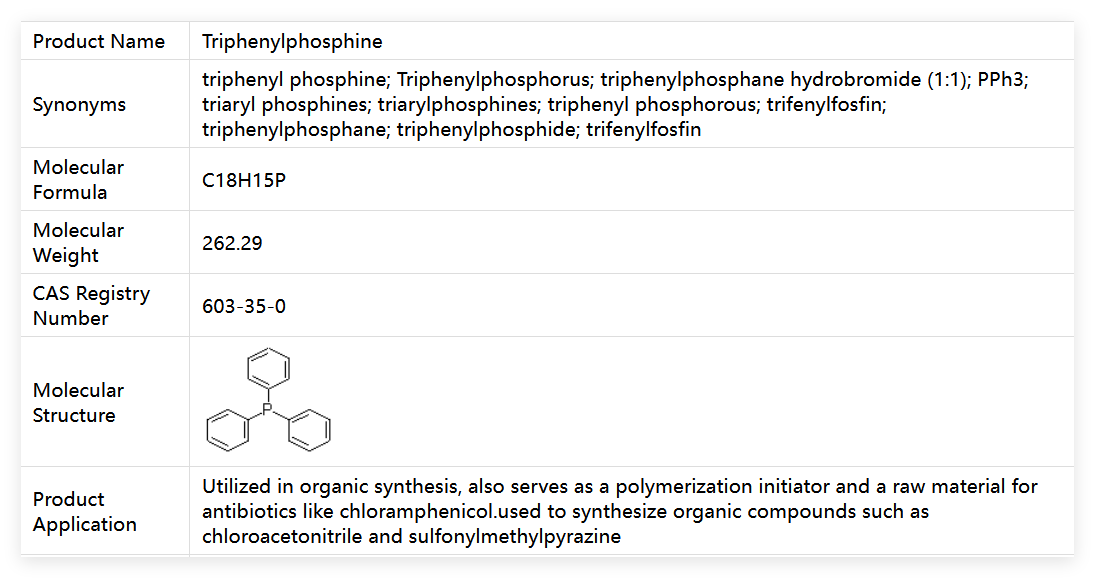
Triphenylphosphine (abbreviated as TPP) is a core organophosphorus compound with CAS No. 603-35-0, molecular formula C₁₈H₁₅P, and molecular weight 262.285. It is a high-quality industrial-grade reagent. The product appears as white powder or flaky crystals with no obvious odor, exhibiting excellent chemical stability under conventional conditions, requiring only avoidance of direct contact with strong oxidants. Its hazard labels comply with GHS standards, marked with GHS07 (Irritation) and GHS08 (Health Hazards), with the signal word "Warning". It is classified under National Standard Number 61861 (toxic substance classification) and must be used in professional operating scenarios. It is an indispensable key reagent in fields such as organic synthesis, pharmaceutical manufacturing, and catalytic reactions.
As a high-efficiency reducing agent, its core advantage lies in the formation of thermodynamically stable triphenylphosphine oxide (Ph₃PO) during reactions. This reaction has strong driving force, enabling precise deoxygenation conversion of substances such as hydrogen peroxide and ozonides to selectively produce target products like alcohols, aldehydes, and ketones. Meanwhile, as a transition metal ligand, it can form stable complexes with metals such as palladium, rhodium, and nickel (e.g., the classic catalyst Pd(PPh₃)₄). When catalyzing coupling reactions, it operates under mild conditions, achieving a carbon-carbon bond formation yield of over 90%, which meets the requirements of precision synthesis.
The product has a density of 1.132 (relative to water), a stable melting point of 79-81°C, a boiling point of 360.0±11.0°C (at 760 mmHg), a flash point of 181.7°C, and low volatility (vapor pressure of only 0.0±0.8 mmHg at 25°C). It can be safely operated within the temperature range of conventional laboratories or industrial workshops. Its solubility exhibits the characteristic of "insoluble in water, easily soluble in ether/benzene", facilitating the separation of reaction systems and post-treatment, and reducing purification difficulty.
Under the conditions of cool and ventilated storage with sealed packaging, it can be stored for a long time without polymerization or deterioration. It only needs to be kept away from ignition sources, heat sources, and oxidants to prevent thermal decomposition into phosphine and phosphorus oxide fumes. Under conventional storage conditions, the purity and activity of the product can remain stable, without the need for special low-temperature or inert gas protection, reducing storage costs.
Relying on its versatility, triphenylphosphine is widely used in four core fields: pharmaceuticals, pesticides, materials, and organic synthesis. The specific application scenarios are as follows:
- It serves as a core raw material for the antibiotic drug clindamycin, participating in the construction of the drug molecular skeleton to ensure the purity and efficiency of antibiotic production.
- It is used as a standard sample for "phosphorus content determination" in organic microanalysis, which can calibrate the accuracy of detection instruments and ensure precise control of phosphorus content in pharmaceutical intermediates.
It synthesizes the organophosphorus intermediate "trimethyl phosphite" through transesterification, which is further derived to prepare broad-spectrum insecticides such as dichlorvos, monocrotophos, and phosphamidon. It contributes to agricultural pest and disease control, with high reaction conversion rate and reduced by-product pollution.
- As a stabilizer for synthetic rubber and resins, it can inhibit the aging and degradation of polymer materials during storage or use, extending the service life of products.
- As an antioxidant for polyvinyl chloride (PVC), it prevents oxidative discoloration of PVC during processing due to high temperature, improving the appearance and performance of products.
- It participates in the synthesis of alkyd resins and polyester resins, enhancing the weather resistance and mechanical strength of resins to meet the needs of downstream products such as coatings and adhesives.
- Reduction reactions: Decomposing ozonides to prepare aldehydes and ketones, converting thiiranes to alkenes, and dehalogenating α-bromoketones, etc. These reactions have high selectivity and easy separation of by-products.
- Catalytic reactions: As a ligand for catalysts such as Pd(PPh₃)₄, it promotes coupling reactions between phenylboronic acid and aryl halides, dequaternization of pyridinium salts, Beckmann rearrangement, etc., which is suitable for the synthesis of fine chemical products and pharmaceutical intermediates.
Triphenylphosphine belongs to Toxic Substance Class 6.1 with Dangerous Goods Transport Code 3077. Its transportation must strictly comply with national dangerous goods transportation regulations. The specific requirements are as follows:
It is packaged in polyethylene or glass bottles with good sealing performance, covered with anti-collision cartons, and filled with buffer materials (such as foam particles) inside the cartons to prevent leakage caused by packaging damage during transportation. The outer packaging must be clearly marked with "Toxic Substance", "Keep Away from Ignition Sources" labels, CAS No., manufacturer information, and emergency contact information.
- Mixed loading and transportation with oxidants and food chemicals is prohibited. The carriage must be independently partitioned to avoid chemical reactions caused by contact between goods.
- Transport vehicles must have dangerous goods transportation qualifications, equipped with GPS positioning systems and emergency rescue equipment (such as fire extinguishers, absorbent cotton, and chemical protective gloves).
- Transportation in high-temperature, direct sunlight, rainy, or snowy weather should be avoided. Sunshade measures must be taken for transportation in summer, and antifreeze measures should be paid attention to in winter (although the product has a low melting point and low temperature does not affect its stability, it is necessary to prevent packaging from freezing and cracking).
In case of leakage during transportation, the vehicle must be stopped immediately and the contaminated area isolated. The driver should wear N95 dust masks, chemical protective clothing, and chemical-resistant gloves. Small amounts of leakage should be adsorbed with dry sand and put into sealed bags. For large amounts of leakage, professional dangerous goods disposal institutions should be contacted. Random disposal or direct flushing with water is strictly prohibited (the product is insoluble in water, and flushing may cause pollution diffusion).

Triphenylphosphine (abbreviated as TPP) is a core organophosphorus compound with CAS No. 603-35-0, molecular formula C₁₈H₁₅P, and molecular weight 262.285. It is a high-quality industrial-grade reagent. The product appears as white powder or flaky crystals with no obvious odor, exhibiting excellent chemical stability under conventional conditions, requiring only avoidance of direct contact with strong oxidants. Its hazard labels comply with GHS standards, marked with GHS07 (Irritation) and GHS08 (Health Hazards), with the signal word "Warning". It is classified under National Standard Number 61861 (toxic substance classification) and must be used in professional operating scenarios. It is an indispensable key reagent in fields such as organic synthesis, pharmaceutical manufacturing, and catalytic reactions.
As a high-efficiency reducing agent, its core advantage lies in the formation of thermodynamically stable triphenylphosphine oxide (Ph₃PO) during reactions. This reaction has strong driving force, enabling precise deoxygenation conversion of substances such as hydrogen peroxide and ozonides to selectively produce target products like alcohols, aldehydes, and ketones. Meanwhile, as a transition metal ligand, it can form stable complexes with metals such as palladium, rhodium, and nickel (e.g., the classic catalyst Pd(PPh₃)₄). When catalyzing coupling reactions, it operates under mild conditions, achieving a carbon-carbon bond formation yield of over 90%, which meets the requirements of precision synthesis.
The product has a density of 1.132 (relative to water), a stable melting point of 79-81°C, a boiling point of 360.0±11.0°C (at 760 mmHg), a flash point of 181.7°C, and low volatility (vapor pressure of only 0.0±0.8 mmHg at 25°C). It can be safely operated within the temperature range of conventional laboratories or industrial workshops. Its solubility exhibits the characteristic of "insoluble in water, easily soluble in ether/benzene", facilitating the separation of reaction systems and post-treatment, and reducing purification difficulty.
Under the conditions of cool and ventilated storage with sealed packaging, it can be stored for a long time without polymerization or deterioration. It only needs to be kept away from ignition sources, heat sources, and oxidants to prevent thermal decomposition into phosphine and phosphorus oxide fumes. Under conventional storage conditions, the purity and activity of the product can remain stable, without the need for special low-temperature or inert gas protection, reducing storage costs.
Relying on its versatility, triphenylphosphine is widely used in four core fields: pharmaceuticals, pesticides, materials, and organic synthesis. The specific application scenarios are as follows:
- It serves as a core raw material for the antibiotic drug clindamycin, participating in the construction of the drug molecular skeleton to ensure the purity and efficiency of antibiotic production.
- It is used as a standard sample for "phosphorus content determination" in organic microanalysis, which can calibrate the accuracy of detection instruments and ensure precise control of phosphorus content in pharmaceutical intermediates.
It synthesizes the organophosphorus intermediate "trimethyl phosphite" through transesterification, which is further derived to prepare broad-spectrum insecticides such as dichlorvos, monocrotophos, and phosphamidon. It contributes to agricultural pest and disease control, with high reaction conversion rate and reduced by-product pollution.
- As a stabilizer for synthetic rubber and resins, it can inhibit the aging and degradation of polymer materials during storage or use, extending the service life of products.
- As an antioxidant for polyvinyl chloride (PVC), it prevents oxidative discoloration of PVC during processing due to high temperature, improving the appearance and performance of products.
- It participates in the synthesis of alkyd resins and polyester resins, enhancing the weather resistance and mechanical strength of resins to meet the needs of downstream products such as coatings and adhesives.
- Reduction reactions: Decomposing ozonides to prepare aldehydes and ketones, converting thiiranes to alkenes, and dehalogenating α-bromoketones, etc. These reactions have high selectivity and easy separation of by-products.
- Catalytic reactions: As a ligand for catalysts such as Pd(PPh₃)₄, it promotes coupling reactions between phenylboronic acid and aryl halides, dequaternization of pyridinium salts, Beckmann rearrangement, etc., which is suitable for the synthesis of fine chemical products and pharmaceutical intermediates.
Triphenylphosphine belongs to Toxic Substance Class 6.1 with Dangerous Goods Transport Code 3077. Its transportation must strictly comply with national dangerous goods transportation regulations. The specific requirements are as follows:
It is packaged in polyethylene or glass bottles with good sealing performance, covered with anti-collision cartons, and filled with buffer materials (such as foam particles) inside the cartons to prevent leakage caused by packaging damage during transportation. The outer packaging must be clearly marked with "Toxic Substance", "Keep Away from Ignition Sources" labels, CAS No., manufacturer information, and emergency contact information.
- Mixed loading and transportation with oxidants and food chemicals is prohibited. The carriage must be independently partitioned to avoid chemical reactions caused by contact between goods.
- Transport vehicles must have dangerous goods transportation qualifications, equipped with GPS positioning systems and emergency rescue equipment (such as fire extinguishers, absorbent cotton, and chemical protective gloves).
- Transportation in high-temperature, direct sunlight, rainy, or snowy weather should be avoided. Sunshade measures must be taken for transportation in summer, and antifreeze measures should be paid attention to in winter (although the product has a low melting point and low temperature does not affect its stability, it is necessary to prevent packaging from freezing and cracking).
In case of leakage during transportation, the vehicle must be stopped immediately and the contaminated area isolated. The driver should wear N95 dust masks, chemical protective clothing, and chemical-resistant gloves. Small amounts of leakage should be adsorbed with dry sand and put into sealed bags. For large amounts of leakage, professional dangerous goods disposal institutions should be contacted. Random disposal or direct flushing with water is strictly prohibited (the product is insoluble in water, and flushing may cause pollution diffusion).