+86-13961672821
+86-510-86268020
trust@hi2000.com
trust@hi2000.com
Room 807,No.169 Changjiang road,Huifu plaza,New centre,Jiangyin,Jiangsu China



| Availability: | |
|---|---|








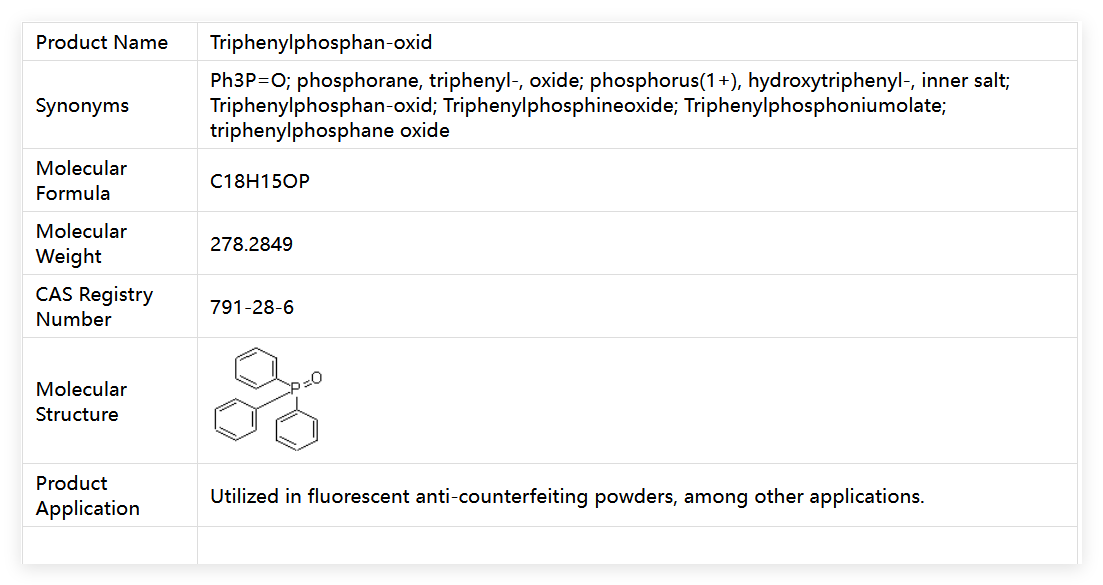
Triphenylphosphine Oxide (chemical name: Triphenylphosphine Oxide, abbreviated as TPPO in English) is an organophosphorus compound with both functionality and stability, and also a key chemical reagent in industrial production and scientific research experiments. Its core identification information is clear: CAS No. 791-28-6, EC No. 212-338-8, RTECS No. SZ1676000, molecular formula C₁₈H₁₅OP, molecular weight 278.285, and exact mass up to 278.086060.
In terms of appearance, the product is in the form of regular white crystals under normal conditions, with uniform crystalline morphology and no obvious impurity particles; in some specific preparation scenarios (such as high-purity purification or mixing with a small amount of auxiliary materials), it may also present a light brown-gray crystalline form, but this does not affect its core performance. The product has no pungent odor, stable physical properties, and is easy to measure and feed, making it a basic raw material with strong adaptability in various chemical processes.
- Excellent Thermal Stability: The density is 1.2±0.1 g/cm³ (the measured value is approximately 1.212 g/cm³ at 25℃/4℃), the melting point range is accurately controlled between 150-157℃ (conforming to the standard value recorded in the literature), the boiling point is 462.9±18.0℃ under the standard pressure of 760 mmHg, and approximately 360℃ under normal pressure. The closed-cup flash point reaches 180℃. Even in medium-low temperature reaction or storage environments, it is not prone to property changes such as melting and decomposition, reducing process risks.
- Strong Controllability of Solubility: Slightly soluble in water, with the logarithm of octanol-water partition coefficient (LogP) being 2.87. This "limited solubility" characteristic makes it highly advantageous in scenarios such as extraction and phase transfer reactions—it can not only achieve dissolution and reaction in the target system but also complete purification through simple phase separation operations, reducing subsequent processing costs.
- High Adaptability of Chemical Inertness: It has stable properties under normal temperature and pressure, and only needs to avoid direct contact with strong oxidants. There is no need for complex inert gas protection or low-temperature storage, resulting in low difficulty in daily use and maintenance, and it is suitable for most conventional chemical production environments.
The product has a clear risk level through GHS classification (signal word: Warning). Although there are risks such as "harmful if swallowed", "skin/eye irritation", "may cause respiratory irritation", and "harmful to aquatic organisms", the corresponding protective measures and emergency treatment plans are mature (such as wearing nitrile rubber gloves and protective glasses). Moreover, its acute toxicity is relatively low (the oral LD50 in mice is 1380 mg/kg). As long as standard operating procedures are followed, the use risks can be effectively controlled.
| Item | Parameter Details | Remarks |
|---------------------|---------------------------------------------------|----------------------------------|
| CAS No. | 791-28-6 | Internationally universal chemical identification |
| Molecular Formula | C₁₈H₁₅OP | Stable molecular structure, containing 18 C, 15 H, 1 O, and 1 P atoms |
| Molecular Weight | 278.285 | Exact mass: 278.086060 |
| Appearance | White crystals (or light brown-gray crystals) | Odorless, good crystallinity |
| Density | 1.2±0.1 g/cm³ (at 25℃/4℃) | Measured value: approximately 1.212 g/cm³ |
| Melting Point | 150-157℃ | Conforming to the value recorded in the literature (lit.) |
| Boiling Point | 462.9±18.0℃ (at 760 mmHg); 360℃ (under normal pressure) | Parameter differences under different pressure conditions |
| Flash Point | 180℃ (closed-cup) | Good fire safety performance |
| Solubility | Slightly soluble in water; LogP=2.87 | Suitable for phase distribution process requirements |
| Stability | Stable under normal temperature and pressure; avoid contact with strong oxidants | No complex storage conditions required |
As the core raw material of fluorescent anti-counterfeiting powder, the chemical structure of triphenylphosphine oxide can endow the anti-counterfeiting powder with excellent fluorescence excitation and emission performance. Under the irradiation of light with a specific wavelength, it can emit clear and stable fluorescent signals, and has strong light aging resistance, which is not easy to cause fluorescence attenuation due to long-term light exposure. Therefore, it is widely used in high-end anti-counterfeiting scenarios, such as anti-counterfeiting marks for important documents (passports, ID cards), anti-counterfeiting coatings for luxury packaging (such as luggage, wine labels), and anti-counterfeiting pattern preparation for large-value bills and securities, effectively enhancing the recognition and anti-counterfeiting threshold of anti-counterfeiting marks.
- Application as Catalyst: In the synthesis reaction of fine chemical products (such as special epoxy resins and high-performance plastic additives), it can be used as a coordination catalyst. By forming a stable coordination structure with metal ions, it promotes the directional progress of the reaction, reduces the generation of by-products, and improves the purity (purity can reach more than 98%) and reaction yield of the target product.
- Application as Extractant: Relying on its specific solubility characteristics, it acts as an extractant in processes such as rare metal separation (such as extraction of precious metals palladium and platinum) and organic pollutant purification. It can accurately combine with target substances and realize phase transfer, with high separation efficiency. Moreover, it is easy to desorb from target substances in the later stage and can be recycled, reducing industrial costs.
As an important pharmaceutical intermediate, triphenylphosphine oxide plays a key role in the synthesis process of various clinical drugs (such as some antibacterial drugs and anti-inflammatory drugs). The phosphorus-oxygen bond in its molecule can provide active sites, provide necessary structural support for the construction of drug molecules, help form a molecular skeleton with pharmacological activity, and at the same time ensure the reaction selectivity in the drug synthesis process, reducing the impact of impurities on the final drug efficacy. It is an indispensable raw material in the pharmaceutical and chemical field.
It is only for scientific research purposes (not used as civil drugs or household spare reagents) and is often used in experimental projects in chemical laboratories, such as the study of the properties of organophosphorus compounds, the exploration of reaction mechanisms (such as the verification of Wittig reaction mechanism), and the performance testing of new catalysts. Its stable physical and chemical properties and clear chemical activity can provide reliable experimental data support for researchers, contributing to the basic research and technological breakthroughs in fields such as organic chemistry and material chemistry.

Triphenylphosphine Oxide (chemical name: Triphenylphosphine Oxide, abbreviated as TPPO in English) is an organophosphorus compound with both functionality and stability, and also a key chemical reagent in industrial production and scientific research experiments. Its core identification information is clear: CAS No. 791-28-6, EC No. 212-338-8, RTECS No. SZ1676000, molecular formula C₁₈H₁₅OP, molecular weight 278.285, and exact mass up to 278.086060.
In terms of appearance, the product is in the form of regular white crystals under normal conditions, with uniform crystalline morphology and no obvious impurity particles; in some specific preparation scenarios (such as high-purity purification or mixing with a small amount of auxiliary materials), it may also present a light brown-gray crystalline form, but this does not affect its core performance. The product has no pungent odor, stable physical properties, and is easy to measure and feed, making it a basic raw material with strong adaptability in various chemical processes.
- Excellent Thermal Stability: The density is 1.2±0.1 g/cm³ (the measured value is approximately 1.212 g/cm³ at 25℃/4℃), the melting point range is accurately controlled between 150-157℃ (conforming to the standard value recorded in the literature), the boiling point is 462.9±18.0℃ under the standard pressure of 760 mmHg, and approximately 360℃ under normal pressure. The closed-cup flash point reaches 180℃. Even in medium-low temperature reaction or storage environments, it is not prone to property changes such as melting and decomposition, reducing process risks.
- Strong Controllability of Solubility: Slightly soluble in water, with the logarithm of octanol-water partition coefficient (LogP) being 2.87. This "limited solubility" characteristic makes it highly advantageous in scenarios such as extraction and phase transfer reactions—it can not only achieve dissolution and reaction in the target system but also complete purification through simple phase separation operations, reducing subsequent processing costs.
- High Adaptability of Chemical Inertness: It has stable properties under normal temperature and pressure, and only needs to avoid direct contact with strong oxidants. There is no need for complex inert gas protection or low-temperature storage, resulting in low difficulty in daily use and maintenance, and it is suitable for most conventional chemical production environments.
The product has a clear risk level through GHS classification (signal word: Warning). Although there are risks such as "harmful if swallowed", "skin/eye irritation", "may cause respiratory irritation", and "harmful to aquatic organisms", the corresponding protective measures and emergency treatment plans are mature (such as wearing nitrile rubber gloves and protective glasses). Moreover, its acute toxicity is relatively low (the oral LD50 in mice is 1380 mg/kg). As long as standard operating procedures are followed, the use risks can be effectively controlled.
| Item | Parameter Details | Remarks |
|---------------------|---------------------------------------------------|----------------------------------|
| CAS No. | 791-28-6 | Internationally universal chemical identification |
| Molecular Formula | C₁₈H₁₅OP | Stable molecular structure, containing 18 C, 15 H, 1 O, and 1 P atoms |
| Molecular Weight | 278.285 | Exact mass: 278.086060 |
| Appearance | White crystals (or light brown-gray crystals) | Odorless, good crystallinity |
| Density | 1.2±0.1 g/cm³ (at 25℃/4℃) | Measured value: approximately 1.212 g/cm³ |
| Melting Point | 150-157℃ | Conforming to the value recorded in the literature (lit.) |
| Boiling Point | 462.9±18.0℃ (at 760 mmHg); 360℃ (under normal pressure) | Parameter differences under different pressure conditions |
| Flash Point | 180℃ (closed-cup) | Good fire safety performance |
| Solubility | Slightly soluble in water; LogP=2.87 | Suitable for phase distribution process requirements |
| Stability | Stable under normal temperature and pressure; avoid contact with strong oxidants | No complex storage conditions required |
As the core raw material of fluorescent anti-counterfeiting powder, the chemical structure of triphenylphosphine oxide can endow the anti-counterfeiting powder with excellent fluorescence excitation and emission performance. Under the irradiation of light with a specific wavelength, it can emit clear and stable fluorescent signals, and has strong light aging resistance, which is not easy to cause fluorescence attenuation due to long-term light exposure. Therefore, it is widely used in high-end anti-counterfeiting scenarios, such as anti-counterfeiting marks for important documents (passports, ID cards), anti-counterfeiting coatings for luxury packaging (such as luggage, wine labels), and anti-counterfeiting pattern preparation for large-value bills and securities, effectively enhancing the recognition and anti-counterfeiting threshold of anti-counterfeiting marks.
- Application as Catalyst: In the synthesis reaction of fine chemical products (such as special epoxy resins and high-performance plastic additives), it can be used as a coordination catalyst. By forming a stable coordination structure with metal ions, it promotes the directional progress of the reaction, reduces the generation of by-products, and improves the purity (purity can reach more than 98%) and reaction yield of the target product.
- Application as Extractant: Relying on its specific solubility characteristics, it acts as an extractant in processes such as rare metal separation (such as extraction of precious metals palladium and platinum) and organic pollutant purification. It can accurately combine with target substances and realize phase transfer, with high separation efficiency. Moreover, it is easy to desorb from target substances in the later stage and can be recycled, reducing industrial costs.
As an important pharmaceutical intermediate, triphenylphosphine oxide plays a key role in the synthesis process of various clinical drugs (such as some antibacterial drugs and anti-inflammatory drugs). The phosphorus-oxygen bond in its molecule can provide active sites, provide necessary structural support for the construction of drug molecules, help form a molecular skeleton with pharmacological activity, and at the same time ensure the reaction selectivity in the drug synthesis process, reducing the impact of impurities on the final drug efficacy. It is an indispensable raw material in the pharmaceutical and chemical field.
It is only for scientific research purposes (not used as civil drugs or household spare reagents) and is often used in experimental projects in chemical laboratories, such as the study of the properties of organophosphorus compounds, the exploration of reaction mechanisms (such as the verification of Wittig reaction mechanism), and the performance testing of new catalysts. Its stable physical and chemical properties and clear chemical activity can provide reliable experimental data support for researchers, contributing to the basic research and technological breakthroughs in fields such as organic chemistry and material chemistry.